Toni West, currently a postdoctoral research fellow at the Oden Institute's Willerson Center for Cardiovascular Modelling and Simulation, has been awarded the Ruth L. Kirschstein postdoctoral fellowship from the National Institutes of Health.
This opportunity is designed to support highly promising postdoctoral candidates with the potential to significantly advance the study of health. Dr. West was chosen for her research on uncovering the cellular basis for aortic valve disease. In receiving her fellowship, she became the third NIH postdoctoral fellow in the Willerson Center, alongside Drs. Christian Goodbrake and Dan Howsmon.
The Willerson Center is so named in honor of late American cardiologist, James T Willerson, who was the President Emeritus, Director of Cardiology Research, and Co-Director of the Cullen Cardiovascular Research Laboratories at the Texas Heart Institute.
“These are very competitive awards and speak volumes about Dr. Willerson’s vision, the Oden Institute, and the greater research environment at The University of Texas at Austin, ” said Director of the Willerson Center, Michael Sacks.
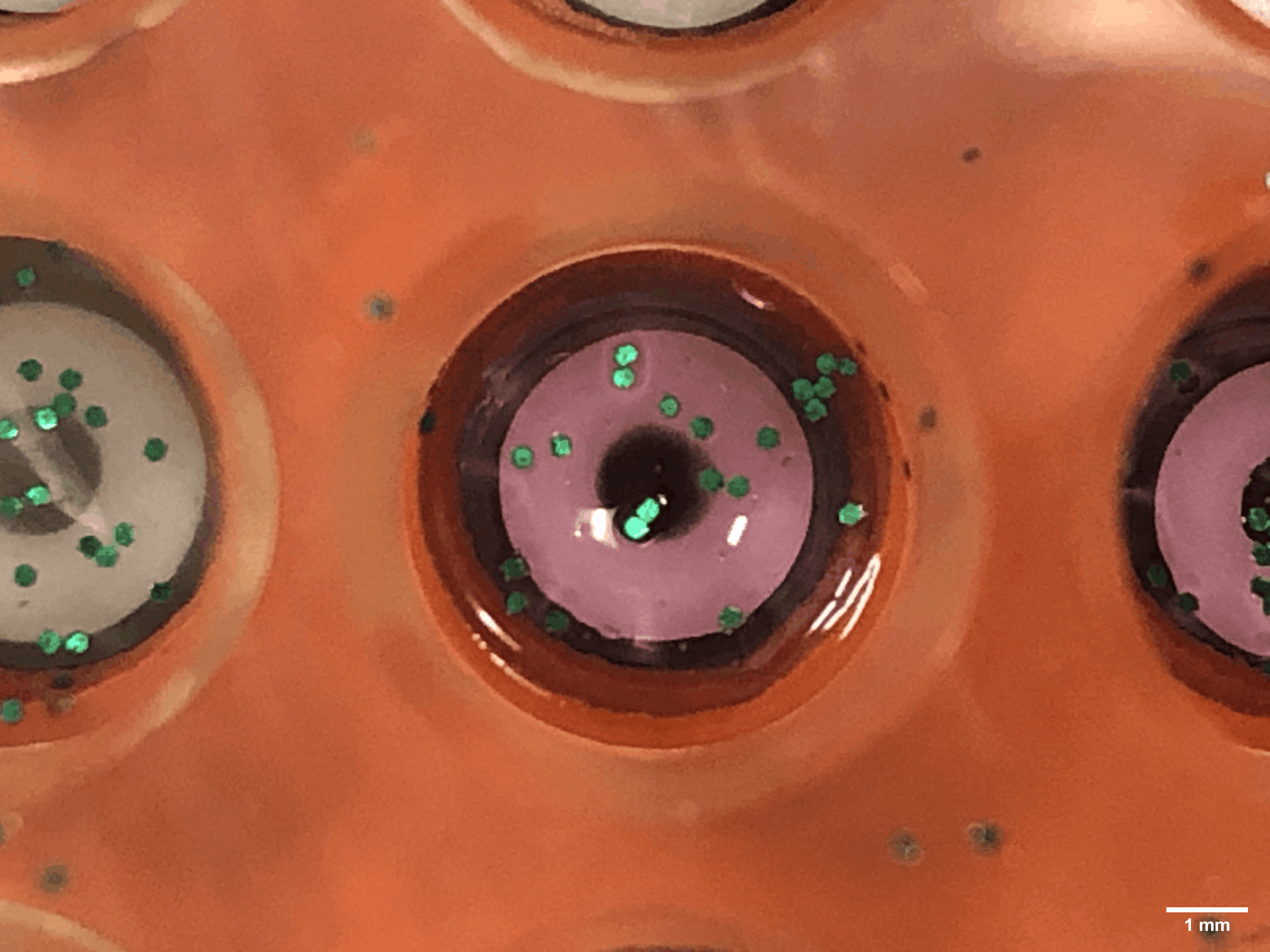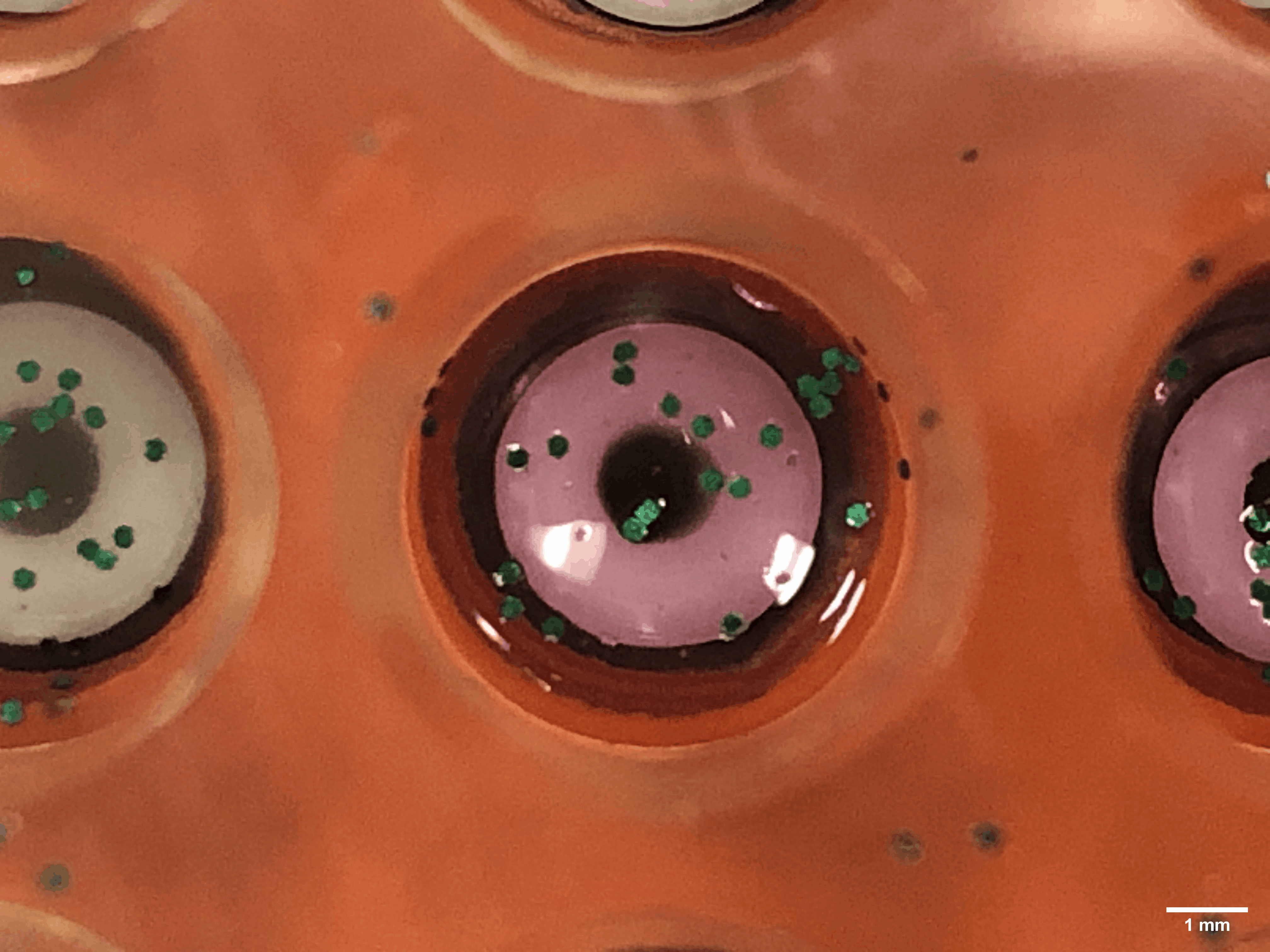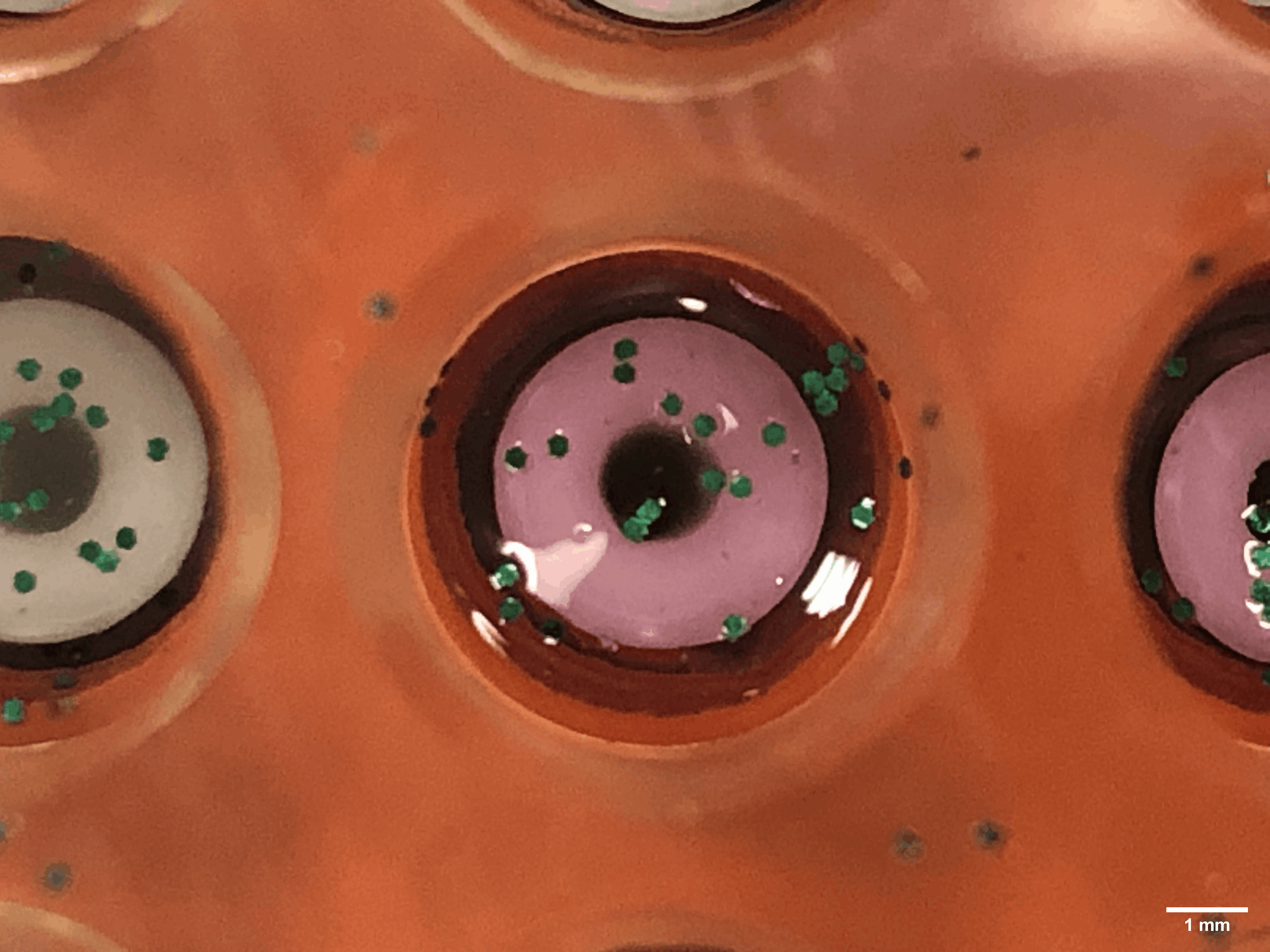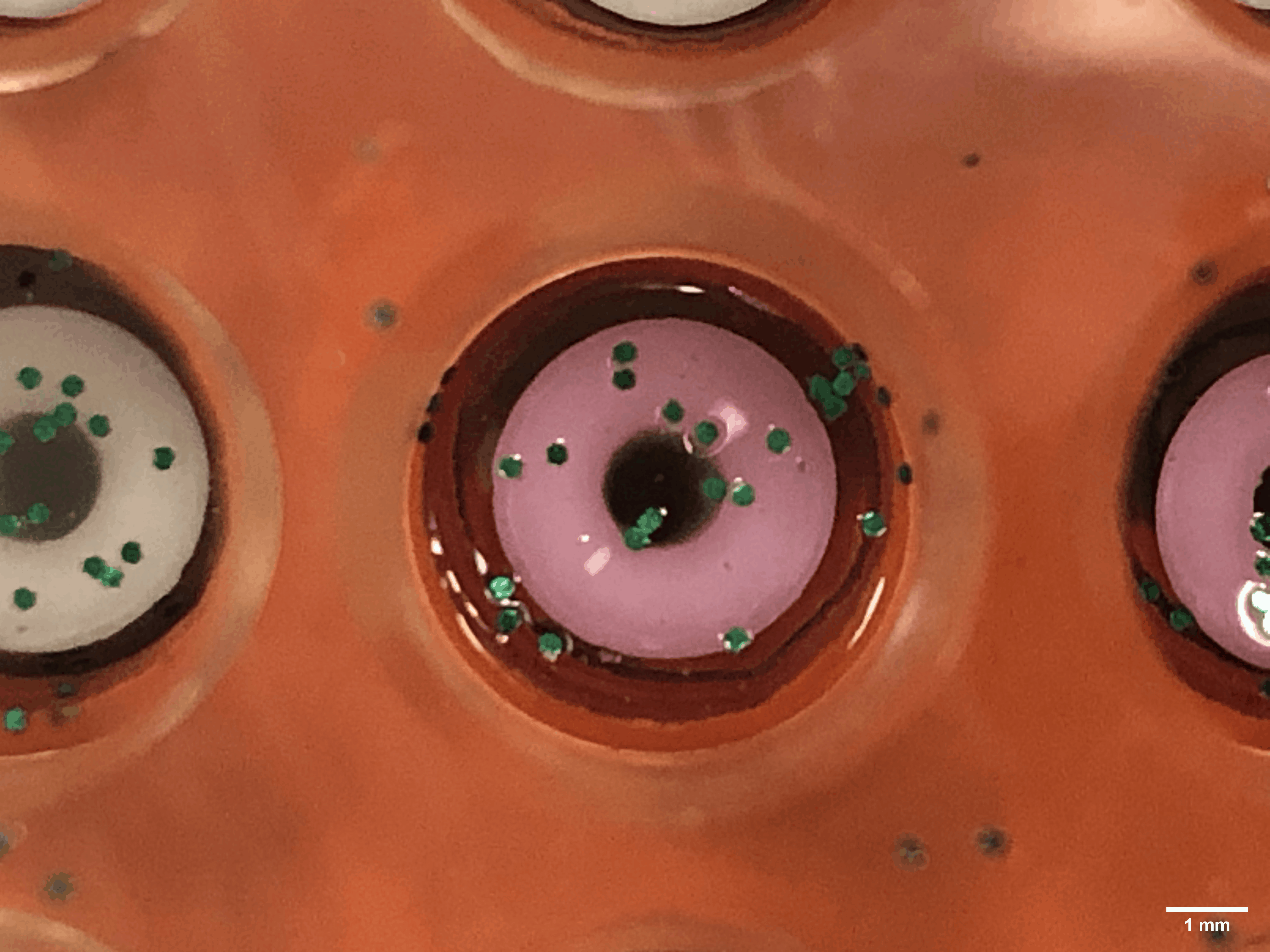
Every time a human heart beats, its aortic valves open and close – over 3 billion times in a typical lifetime. Though impressively durable, aortic valves can become diseased and require surgical replacement – a procedure that continues to have problems with regards to long-term durability.