Unlike machine learning and other statistical approaches that rely on massive datasets and population averages, the Oden Institute team’s models are rooted in biology itself. They incorporate the known physical processes of cancer: how tumor cells grow, move, interact with surrounding tissues, and respond to therapy. These processes are captured mathematically through partial differential equations (a type of equation that describes changes in space and time).
This patient-specific approach means the model doesn’t just look at what worked for most people. Instead, it is tailored to the individual. “If you want to improve how an individual will respond to treatment, then you have to be able to predict how a given intervention will work on that individual,” Yankeelov said.
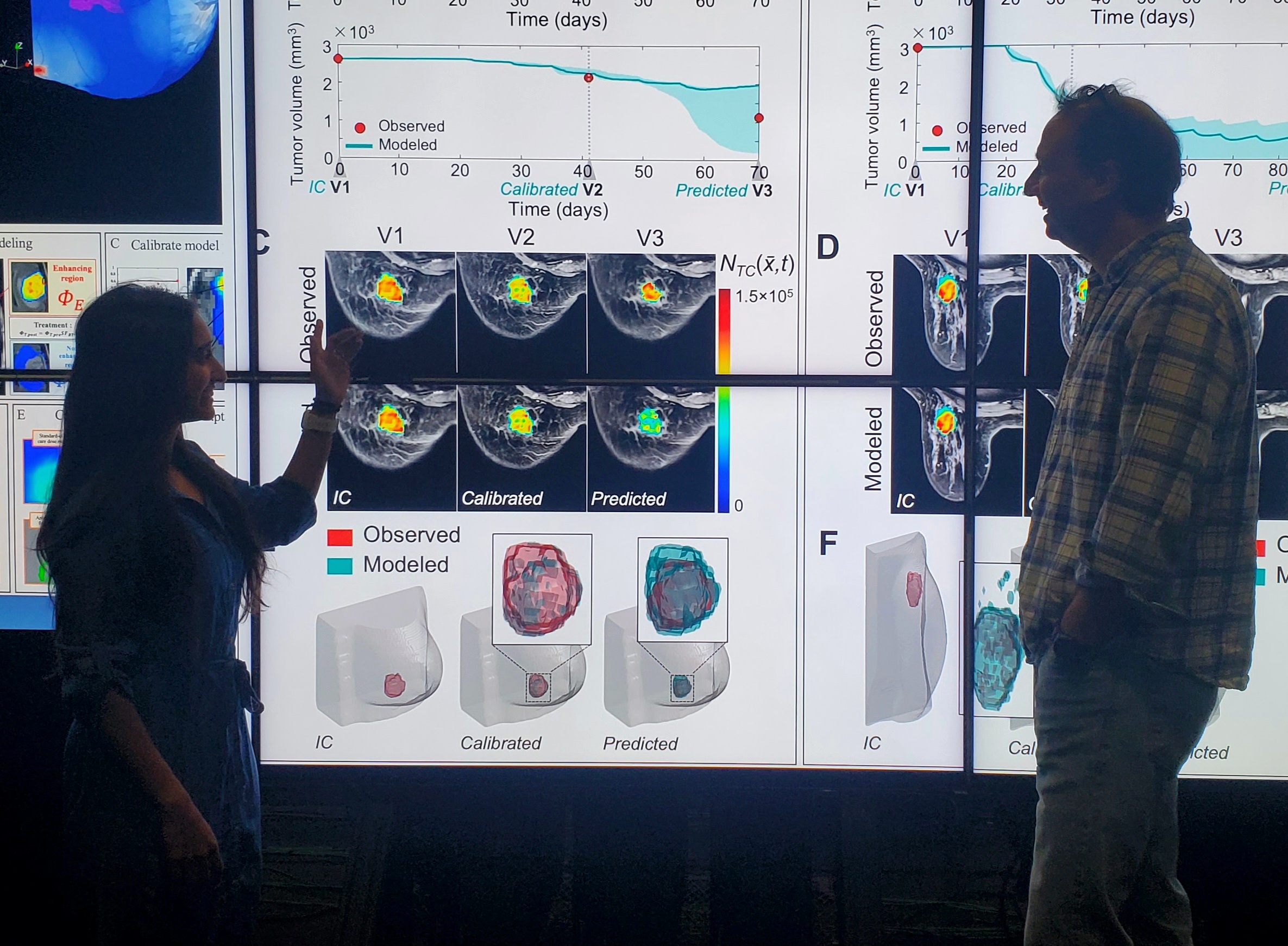
To validate their work, the team analyzed data from 91 breast cancer patients across 10 clinical trial sites, covering three different subtypes of breast cancer. This diversity of data was critical to testing the robustness of the model. “When you get data from 10 different clinical sites, there can be a fair amount of variation in the quality and quantity of data,” Yankeelov explained. “So, if your mathematical model is able to make accurate predictions on data with that degree of variability, then you start to think that you are on to something that captures key features of tumors and what causes them to grow or respond to therapy.”
A key collaborabor on the study, Chengyue Wu, an assistant professor in the Department of Imaging Physics at UT's MD Anderson Cancer Center, noted the long path that brought the team to this point. “It’s quite a journey to develop the mathematical model for breast cancer treatment response and gradually validate it through multiple studies. We started the proof-of-principal development in a small cohort of about 10 cases, then we’ve been refining it and applied it to a single-institutional large cohort. Now we are able to validate its generalizability in the multi-institutional I-SPY2 cohort. It is super exciting to see the continuous growth of evidence and confidence for our model’s ability to make real-world impact in breast cancer care.” Wu is also an Oden Institute alumna.